
 Flash News
Flash News
"SP gets 84 mandates", how did Open Data predict the results of May 11?
Tirana Court Sentences Dan Hutra, Serial Killer of Women, to Life in Prison
Shkëlqim Shehu wins mandate, most voted in Kukës from DP
About 80 thousand envelopes from the diaspora are being counted, results so far
Foreign exchange/ How much foreign currencies are bought and sold today
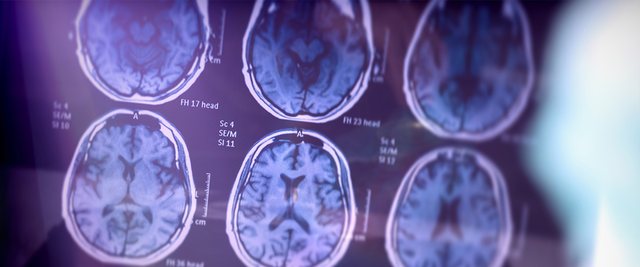
The US Food and Drug Administration (FDA) recently approved a new drug that appears to slow the progression of Alzheimer's disease. The FDA said Leqembi, known chemically as lecanemab, will be given to patients with mild or early stages of the degenerative brain disease. The drug is the first of its kind to convincingly show researchers that it can slow the decline of memory and thinking. The drug is expected to cost about $25,000 a year for a single patient.

The agency said its decision was based on a trial of 856 Alzheimer's patients. In late September, Eisai and Biogen, the companies that developed the drug, announced that a phase 3 clinical trial with 1,795 patients found that Leqembi slowed cognitive decline in people who took it by 27% after 18 months. "This medicine is not a cure. It doesn't stop people from getting worse, but it measurably slows the progression of the disease," neurologist Joy Snider said in an interview with NBC. "This could mean someone could have an extra six months to a year to drive." Medical experts said the drug comes with downsides, including potential side effects such as brain swelling and the need for twice-monthly infusions. The challenge in getting the drug to patients is the next obstacle, as it may be months before it is distributed and insurers must decide whether and how to cover the drug's high cost. According to health experts, nearly six million Americans suffer from Alzheimer's and millions more suffer from the disease worldwide.

Alzheimer's disease gradually attacks the areas of the brain needed for memory, reasoning, communication and everyday tasks. Scientists have developed a blood test to diagnose Alzheimer's disease without the need for brain scans or painful procedures where a sample of cerebrospinal fluid (CSF) is taken from the lower back. If effective, the test could enable faster diagnosis of the disease, meaning therapies could begin earlier. Alzheimer's is the most common form of dementia, but diagnosis remains difficult, especially during the early stages of the disease.
Latest news



Tirana Court Sentences Dan Hutra, Serial Killer of Women, to Life in Prison
2025-05-14 10:10:55
List of MPs that Elbasan is bringing to the Assembly
2025-05-14 10:01:46
"Zjerm" leads Albania to the Eurovision 2025 final
2025-05-14 09:50:29

New parties open a window to Parliament for the first time
2025-05-14 09:31:45
Rama delivers a speech at Skanderbeg Square today
2025-05-14 09:31:34

Shkëlqim Shehu wins mandate, most voted in Kukës from DP
2025-05-14 09:11:58
Votes for candidates, names from the open list entering the Assembly in Lezha
2025-05-14 09:03:23

How was the vote stolen in London? Hasjani tells it live
2025-05-14 08:37:52
About 80 thousand envelopes from the diaspora are being counted, results so far
2025-05-14 08:30:37
Foreign exchange/ How much foreign currencies are bought and sold today
2025-05-14 08:13:53

4 cars collide in Tirana, after the accident the drivers fight with levers
2025-05-14 07:58:20
Weather forecast for today
2025-05-14 07:40:35
HOROSCOPE/ Here's what the stars have predicted for each sign
2025-05-14 07:20:57
Morning Post/ In 2 lines: What mattered yesterday in Albania
2025-05-14 07:06:32

SP towards full legislative control, what does this mean for democracy?
2025-05-13 22:36:52

'Braçeee, Braçeee', the counter grabs the attention of CEAZ 5, laughter erupts
2025-05-13 21:55:09

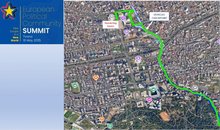
Tirana hosts European leaders on May 16, summit agenda revealed
2025-05-13 21:11:22
Counting closes in Vlora, PS gets 9 mandates, DP remains with 3
2025-05-13 20:59:14
DP representative at the CEC: SP votes in the diaspora, filled by the same hand
2025-05-13 20:50:55

BIRN: European Union concerned about violation of electoral standards in Albania
2025-05-13 20:21:37
A house for a vote? Soft loans for the administration before the elections
2025-05-13 20:10:01
DP-ASHM secures two mandates in Kukës, Isuf Çelaj the most preferred voter
2025-05-13 20:01:22
Poll/ Were the May 11 elections fair?
2025-05-13 19:27:22
Elections 2025/ Counting of diaspora votes continues, results
2025-05-13 19:21:11
Lapaj: We are close to a mandate in Fier, we will take it from the SP
2025-05-13 19:17:33

82 mandates, a reward for what?
2025-05-13 18:36:46
International cyber fraud network hit, seizures and checks in Tirana
2025-05-13 18:17:08








"Electronic Shkodra" takes to the Eurovision 2025 stage tonight
2025-05-13 16:02:42
Discover the 5 reasons to take folic acid even if you are not pregnant.
2025-05-13 15:45:50
Lack of independent media undermined elections, observers say
2025-05-13 15:34:36
The first ballot boxes for candidates in Rrogozhina are being counted.
2025-05-13 15:31:42




Albania has the most underdeveloped agro-industry in the region, Kosovo is first
2025-05-13 14:43:43


Preferential vote counting concludes in CEAZ 69, Skrapar
2025-05-13 14:23:43
The Director of the ARA appears before SPAK
2025-05-13 14:15:18

Berisha calls for protest on May 16: Elections were dictated by criminal gangs
2025-05-13 13:56:19
Aida Hajnaj is excluded from re-running for a second term as head of the BKH
2025-05-13 13:50:26
Accident near 9-year-old school in Korça, car hits student
2025-05-13 13:40:23

Lezha Court releases Elda Hoti's husband
2025-05-13 13:24:50


"Toyota Yaris" file/ SPAK demands 6 years in prison for lawyer Radovan Çela
2025-05-13 12:53:16
Conflict between observers at a CEAZ in Tirana, 7 escorted
2025-05-13 12:44:39
Counting closes in Kamëz, DP leads
2025-05-13 12:37:10

Shkodra, family members find 65-year-old woman hanged
2025-05-13 12:21:03
Alimehmeti: Albania cannot go to Europe with these elections
2025-05-13 12:12:27

Durres Prison 'bastion' of the Socialist Party
2025-05-13 11:49:41
A house in Lezha is engulfed in flames
2025-05-13 11:36:28

Our Putin!
2025-05-13 11:23:36

Kosovo Assembly fails to unblock, 15th session fails too
2025-05-13 10:58:49

Voting ends in Berat/ SP gets 5 mandates, DP-ASHM gets 2
2025-05-13 10:40:00
Kikia: Rama's fourth mandate a precedent for democracy
2025-05-13 10:28:22
Democrats win Kavaja convincingly
2025-05-13 10:21:00


US lifts temporary protection for thousands of Afghans
2025-05-13 09:44:52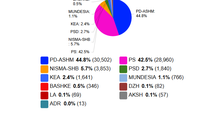
Overturning the result in Lezha, PD-ASHM secures 4 MPs
2025-05-13 09:44:00

Counting suspended in VIP prison, two more ballots found
2025-05-13 09:23:54
European leaders gather in Tirana in search of creative solutions for Ukraine
2025-05-13 09:11:52

Countdown nears, DP seizes lead in Shkodra and Lezha
2025-05-13 08:51:05
Result/ PS wins for the first time in Paskuqan
2025-05-13 08:39:26


Clear skies and cloudy skies, this is the weather forecast for Tuesday
2025-05-13 08:02:42
Morning Post/ In 2 lines: What mattered yesterday in Albania
2025-05-13 07:48:57
