
 Flash News
Flash News
Gunfire in Durres, a 30-year-old man is injured
Accident on Arbri Street, car goes off the road, two injured
Arrests of "Bankers Petrolium", Prosecution provides details: Exported and sold 532 billion lek of oil, caused millions of euros in damage to the state
Ndahet nga jeta tragjikisht në moshën 28-vjeçare ylli i Liverpool, Diogo Jota
Posta e mëngjesit/ Me 2 rreshta: Çfarë pati rëndësi dje në Shqipëri
After numerous accusations, Johnson & Johnson stops the production of baby powder worldwide

Johnson & Johnson (J&J) will stop making and selling talc-based baby powder worldwide, starting next year.
The announcement comes more than two years after the healthcare giant halted sales of the product in the US.
J&J has faced tens of thousands of lawsuits from women who claim the talcum powder contained asbestos and caused them ovarian cancer. But the company reiterated its view that decades of independent research show the product is safe to use.
In the last statement, the brand emphasized once again that its baby powder is safe:
"Our position on the safety of our cosmetic talc remains unchanged. We stand firm after decades of independent scientific analysis by medical experts around the world confirming that Johnson's talc-based baby powder is safe, asbestos-free and cancer-free .”
In 2020, J&J said it would stop selling baby powder in the US and Canada because demand had fallen after what the brand called "misinformation" about the product's safety amid a number of legal issues.
At the time, the firm said it would continue to sell the powder in the rest of the world.
A 2018 investigation by the Reuters news agency alleged that J&J had known for decades that asbestos was present in its talc products. Reuters said internal company records, testimony and other evidence showed that from at least 1971 to the early 2000s, J&J's raw talc and finished powders sometimes tested positive for trace amounts of asbestos.
In response to evidence of asbestos contamination presented in courtrooms, media reports and US lawmakers, the firm has repeatedly denied the allegations.
In October, J&J created a subsidiary, LTL Management, assigning the talc claims to it. The subsidiary later filed for bankruptcy, which terminated the pending lawsuits.
Latest news










Greece imposes fee to visit Santorini, how many euros tourists must pay
2025-07-03 20:50:37
Don't make fun of the highlanders, Elisa!
2025-07-03 20:43:43
Gunfire in Durres, a 30-year-old man is injured
2025-07-03 20:30:52

The recount in Fier cast doubt on the integrity of the vote
2025-07-03 20:09:03




Heatwave has left at least 9 dead this week in Europe
2025-07-03 19:00:01

Oil exploitation, Bankers accused of 20-year fraud scheme
2025-07-03 18:33:52
Three drinks that make you sweat less in the summer
2025-07-03 18:19:35
What we know so far about the deaths of Diogo Jota and his brother André Silva
2025-07-03 18:01:56



Another heat wave is expected to grip Europe
2025-07-03 17:10:58

Accident on Arbri Street, car goes off the road, two injured
2025-07-03 16:45:27

Accused of two murders, England says "NO" to Ilirjan Zeqaj's extradition
2025-07-03 16:25:05





Gaza rescue teams: Israeli forces killed 25 people, 12 in shelters
2025-07-03 15:08:43
Diddy's trial ends, producer denied bail
2025-07-03 15:02:41

Agricultural production costs are rising rapidly, 4.8% in 2024
2025-07-03 14:55:13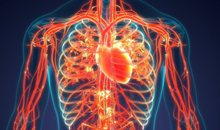
Warning signs of poor blood circulation
2025-07-03 14:49:47
Croatia recommends its citizens not to travel to Serbia
2025-07-03 14:31:19
Berisha: Albania is the blackest stain in Europe for the export of emigrants
2025-07-03 14:20:19


'Ministry of Smoke': Activists Blame Government for Wasteland Fires
2025-07-03 13:59:09

AFF message of condolences for the tragic loss of Diogo Jota and his brother
2025-07-03 13:41:36
Five healthy foods you should add to your diet
2025-07-03 13:30:19






A unique summer season, full of rhythm and rewards for Credins bank customers!
2025-07-03 12:12:20

Fire situation in the country, 29 fires reported in 24 hours
2025-07-03 12:00:04
The constitution of the Kosovo Assembly fails for the 41st time
2025-07-03 11:59:57
The gendering of politics
2025-07-03 11:48:36
The price we pay after the "elections"
2025-07-03 11:25:39

Xhafa: The fire at the Elbasan landfill was deliberately lit to destroy evidence
2025-07-03 11:08:43

The 3 zodiac signs that will have financial growth during July
2025-07-03 10:48:01
Democratic MP talks about the incinerator, Spiropali turns off her microphone
2025-07-03 10:39:24

Ndahet nga jeta tragjikisht në moshën 28-vjeçare ylli i Liverpool, Diogo Jota
2025-07-03 10:21:03
Cocaine trafficking network in Greece, including Albanians, uncovered
2025-07-03 10:10:12



Korreshi: Election manipulation began long before the voting date
2025-07-03 09:39:13
Arrest of Greek customs officer 'paralyzes' vehicle traffic at Qafë Botë
2025-07-03 09:28:41
After Tirana and Fier, the boxes are opened in Durrës today
2025-07-03 09:21:10
Enea Mihaj transfers to the USA, will play as an opponent of Messi and Uzun
2025-07-03 09:10:04

Foreign exchange, the rate at which foreign currencies are sold and bought
2025-07-03 08:53:50
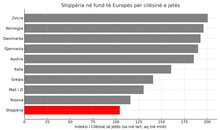
Index, Albania has the worst quality of life in Europe
2025-07-03 08:48:10


Horoscope, what do the stars have in store for you today?
2025-07-03 08:17:05
Clear weather and high temperatures, here's the forecast for this Thursday
2025-07-03 08:00:37
Posta e mëngjesit/ Me 2 rreshta: Çfarë pati rëndësi dje në Shqipëri
2025-07-03 07:46:48



Lufta në Gaza/ Pse Netanyahu do vetëm një armëpushim 60-ditor, jo të përhershëm?
2025-07-02 21:56:08
US suspends some military aid to Ukraine
2025-07-02 21:40:55



Methadone shortage, users return to heroin: We steal to buy it
2025-07-02 20:57:35
Government enters oil market, Rama: New price for consumers
2025-07-02 20:43:30
WHO calls for 50% price hike for tobacco, alcohol and sugary drinks
2025-07-02 20:41:53



